Understanding Obesity: Limitations in Current Measurements and Evolving Treatment Approaches
Overview of Obesity Measurements and Treatments:
Obesity is a significant global health issue characterized by excessive body fat, increasing the risk of cardiovascular disease, type 2 diabetes, and other metabolic disorders. However, traditional measurements of obesity, such as Body Mass Index (BMI) and waist circumference, have notable limitations. In this article, we will explore these limitations in obesity measurements, emphasizing the need for more accurate assessment methods. Additionally, we will discuss evolving treatment approaches aimed at addressing this pressing health concern.
Understanding Body Mass Index (BMI)
BMI Formula:
- Metric: BMI=Weight (kg)/Height (m)2
- Imperial: BMI=Weight (lbs)×703Height (inches)2
BMI Categories:
- Underweight: BMI < 18.5
- Normal weight: BMI 18.5–24.9
- Overweight: BMI 25–29.9
- Obese: BMI ≥ 30
- [bmi_calculator]
Limitations of BMI as a Health Metric in Obesity Measurements
Inability to Differentiate Between Muscle and Fat
- BMI calculates weight relative to height but fails to distinguish between muscle mass and fat mass. Athletes with high muscle density might be inaccurately classified as “overweight” or “obese” (Garrow & Webster, 1985).
Ignores Body Composition
- BMI overlooks factors like bone density and fat distribution, leading to potential misinterpretations of health status (Flegal et al., 2005).
Not Universally Applicable Across Ethnic Groups or Ages
- BMI standards are based on Western populations and may not reflect the body composition of individuals from different ethnic backgrounds or age groups (Bray et al., 2017).
Limited Usefulness in Children and Adolescents
- BMI may misclassify children and adolescents due to the rapid changes in body composition during growth (World Health Organization, 2020).
Variable Health Risks in Individuals
- Metabolically healthy obese (MHO) individuals may not exhibit the same health risks as those with lower BMIs (Bays, 2021).
Does Not Account for Abdominal Fat
- BMI does not measure abdominal or visceral fat, a critical risk factor for heart disease and type 2 diabetes (Bray et al., 2017).
Limitations of Waist Circumference as a Standalone Metric in Obesity Measurements and Treatments
Inability to Differentiate Between Muscle and Fat
- Waist circumference, like BMI, does not distinguish between visceral fat and muscle mass (Garrow & Webster, 1985).
Ignores Overall Body Composition
- This metric provides no insight into overall body fat percentage or muscle mass (Flegal et al., 2005).
No Assessment of Fat Distribution
- Measuring only the waist does not provide information about fat distribution, particularly the amount of harmful visceral fat (World Health Organization, 2020).
Requires Supplementary Health Metrics
- Waist circumference should be used alongside other measures like BMI and body fat percentage for a more comprehensive risk assessment (Bays, 2021).
Alternative Methods for Obesity Measurements
Body Fat Percentage
- Tools such as calipers and bioelectrical impedance scales provide a more accurate measure of body composition than BMI, distinguishing between fat, muscle, and water mass.
Waist-to-Hip Ratio
- This ratio compares waist and hip sizes, offering a better indicator of fat distribution and correlating with cardiovascular disease and type 2 diabetes risks (Bray et al., 2017).
DEXA Scans
- Dual-energy X-ray Absorptiometry (DEXA) scans provide detailed information on fat, muscle, and bone density, considered the gold standard in obesity assessment (Bays, 2021).
Etiology and Pathophysiology of Obesity
Obesity results from an imbalance between energy intake and expenditure, leading to fat accumulation. Contributing factors include genetics, lifestyle habits, and environmental influences. While metabolic conditions like hypothyroidism are sometimes cited, they account for a small proportion of obesity cases. Dietary patterns and sedentary lifestyles are the primary contributors (Flegal et al., 2005).

Metabolism of Carbohydrates, proteins and fats
Treatment Approaches for Obesity
Non-Pharmacological Treatments
Regular Monitoring of BMI, waist circumference, and other health metrics should be measured at least annually to monitor health risks and track progress.
Dietary Changes
- Women: 1,200–1,500 kcal/day
- Men: 1,500–1,800 kcal/day Alternatively, aim for a daily calorie deficit of 500–700 kcal for gradual weight loss of around 1 pound per week. Focus on complex carbohydrates, lean protein, and healthy fats, while reducing refined sugars and trans fats.
Physical Activity Engage in 200–300 minutes of moderate-intensity exercise per week, such as walking, swimming, or cycling.
Lifestyle Modifications to treat obesity
- Quit smoking and reduce alcohol intake.
- Consider intermittent fasting or time-restricted eating to manage calorie intake effectively.
Pharmacological Treatments for Obesity
Pharmacological interventions are recommended for individuals with a BMI ≥ 30 or BMI ≥ 27 with obesity-related comorbidities like type 2 diabetes or hypertension. These medications reduce appetite, enhance satiety, or alter fat absorption.
Prescription Medications for Obesity:
| Drug | Class/MOA | Dose | Side Effects | Safety Notes |
| Diethylpropion | Sympathomimetic/Appetite Suppressant | 25 mg TID | Constipation, dry mouth, insomnia | Caution in patients with liver impairment |
| Phentermine | Sympathomimetic/Appetite Suppressant | 15–37.5 mg daily | Increased heart rate, insomnia, dry mouth | Short-term use; potential for abuse |
| Phentermine + Topiramate SR | Noradrenergic + GABA Enhancer | 3.75–23 mg daily | Dry mouth, constipation, cognitive issues | Contraindicated in pregnancy and glaucoma |
| Bupropion + Naltrexone (Contrave) | Dopamine/Norepinephrine Reuptake Inhibitor + Opioid Antagonist | 8 mg/90 mg, titrate to 2 tabs BID | Nausea, headache, insomnia | Avoid in patients with uncontrolled hypertension |
| Orlistat | Lipase Inhibitor | 120 mg TID with meals | GI disturbances (flatulence, diarrhea) | Separate from fat-soluble vitamins |
| Semaglutide (Wegovy, Ozempic) | GLP-1 Agonist | 0.25 mg–1 mg SC weekly | Nausea, diarrhea, constipation | Approved for obesity and type 2 diabetes |
| Liraglutide (Saxenda) | GLP-1 Agonist | 0.75 mg to 4.0 mg daily SC | Nausea, vomiting, GI discomfort | Approved for long-term weight management |
| Tirzepatide (Mounjaro) | GLP-1 & GIP Agonist | 2.5 mg, 5.0 mg weekly, adjust based on weight loss | Nausea, vomiting, diarrhea | Approved for type 2 diabetes, weight loss efficacy under study |
| Mazindol | Sympathomimetic Appetite Suppressant | 1-3 mg daily | Dry mouth, constipation, increased BP | Short-term use; restricted in many regions |
| Setmelanotide (Imcivree) | MC4 Receptor Agonist | Dose depends on genetic obesity syndrome | Skin darkening, injection-site pain | Approved for genetic obesity syndromes like POMC deficiency |
| Plenity | Non-Systemic, Hydrogel-based | 2.4 grams (3 capsules) twice daily | Abdominal distention, nausea, constipation | FDA-approved for weight management in adults, not absorbed systemically |


Tirzepatide, marketed under brand names such as Mounjaro and Zepbound, is a novel medication developed by Eli Lilly and Company. It functions as a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, enhancing insulin secretion and reducing glucagon levels in a glucose-dependent manner. This dual action contributes to improved glycemic control and significant weight reduction in individuals with type 2 diabetes and obesity.
| Feature | Mounjaro (Tirzepatide) | Ozempic (Semaglutide) | Liraglutide (Saxenda/Victoza) |
| Drug Action | Activates GLP-1 & GIP receptors | Activates GLP-1 receptor only | Activates GLP-1 receptor only |
| Main Uses | Type 2 Diabetes, Weight Loss | Type 2 Diabetes, Weight Loss (as Wegovy) | Type 2 Diabetes (Victoza), Weight Loss (Saxenda) |
| Administration | Injection only (weekly) | Injection (weekly) or Oral (Rybelsus) | Injection only (daily) |
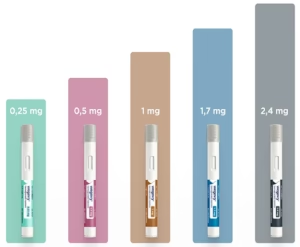
| Dosing | 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg, 15mg (weekly) | 0.25mg, 0.5mg, 1mg, 2mg (weekly) | Victoza: 0.6mg, 1.2mg, 1.8mg (daily) Saxenda: 0.6mg to 3mg (daily) |
| Side Effects | Nausea, diarrhea, reduced appetite, pancreatitis risk | Nausea, diarrhea, vomiting, pancreatitis risk, retinopathy, angioedema | Nausea, vomiting, diarrhea, pancreatitis, gallbladder issues, increased heart rate |
| Risk of Hypoglycemia | Lower risk | Higher risk, especially with insulin | Can occur, especially with insulin or sulfonylureas |
| Storage | Refrigerated but stable at room temp for a short time | Refrigerated but stable at room temp for 6 weeks | Refrigerated but stable at room temp for 30 days |
| Misuse Risk | No major reports | Fake Ozempic pens found | Fake Saxenda pens found |
| Approved for Weight Loss | Yes | Yes (as Wegovy, not Ozempic) | Yes (Saxenda) |
Indications of Tirzepatide:
- Type 2 Diabetes Mellitus: Tirzepatide is approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
- Chronic Weight Management: It is also indicated for chronic weight management in adults with obesity or overweight with at least one weight-related comorbidity, in conjunction with a reduced-calorie diet and increased physical activity.
Mechanism of Action of Tirzepatide:
Tirzepatide mimics the activity of GIP and GLP-1, leading to:
- Enhanced insulin secretion in response to meals.
- Suppression of glucagon release.
- Delayed gastric emptying.
- Reduced food intake.
These effects collectively contribute to improved blood glucose levels and weight loss.
Dosage and Administration:
Tirzepatide is administered via subcutaneous injection once weekly, with dosing individualized based on patient response and tolerability. It is available in multiple strengths to accommodate titration.
Efficacy:
Clinical trials have demonstrated significant benefits of tirzepatide:
- Glycemic Control: In the SURPASS clinical trial program, tirzepatide led to substantial reductions in HbA1c levels compared to other antidiabetic agents.
- Weight Reduction: The SURMOUNT-1 trial reported mean weight reductions up to 20.9% over 72 weeks in individuals without diabetes.
Safety Profile:
Common adverse events of tirzepatide include gastrointestinal symptoms such as nausea, vomiting, and diarrhea, which are generally mild to moderate in severity. There is a boxed warning regarding the potential risk of thyroid C-cell tumors, based on findings in animal studies. Tirzepatide is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2.
Recent Clinical Trial Information:
- Diabetes Prevention: A recent study indicated that tirzepatide reduced the risk of developing type 2 diabetes by 94% in adults with prediabetes and obesity or overweight.
- Weight Loss Comparison: In a head-to-head trial, tirzepatide (Zepbound) demonstrated superior weight loss compared to semaglutide (Wegovy) in individuals with obesity or overweight and at least one comorbidity.
- UK Government Initiative: The UK government, in collaboration with Eli Lilly, has launched a £300 million initiative to conduct weight-loss drug trials focusing on tirzepatide (Mounjaro) in Manchester. These trials aim to evaluate the drug’s efficacy and inform potential large-scale rollout within the NHS.
Obesity Measurements and Treatments: Advancing with Tirzepatide
Obesity is a growing global health challenge that demands accurate measurements and innovative treatments. Tirzepatide, a dual GIP and GLP-1 receptor agonist, is emerging as a game-changer in obesity management.
Key Clinical Trial Insights
- SURMOUNT-2 Trial: Tirzepatide reduced body weight by 15.7% at its highest dose, with 86.4% of participants achieving at least a 5% weight reduction.
- Diabetes Prevention Study: A 94% reduction in diabetes risk was observed in participants with obesity or overweight. Weight loss averaged 22.9%.
- Long-Term Weight Maintenance: The SURMOUNT-4 trial showed 80% weight loss retention over 88 weeks, highlighting tirzepatide’s sustainability.
Beyond Weight Loss
Tirzepatide addresses obesity-related conditions, such as improving liver health in patients with Metabolic Dysfunction–Associated Steatohepatitis (MASH) and slowing kidney function decline in diabetes.
Why It Matters
Accurate obesity measurements and treatments like tirzepatide are crucial for tackling obesity and its associated health risks. This innovative therapy provides a comprehensive solution, offering hope to millions struggling with weight-related issues.
These developments underscore tirzepatide’s potential as a transformative therapy in managing type 2 diabetes and obesity.
Investigational Drugs for Weight Loss:
Tesofensine (not FDA-approved) shows promise in clinical trials as a triple monoamine reuptake inhibitor, aiding in appetite suppression. However, side effects like insomnia and increased heart rate are concerns.
Conclusion of Obesity Measurements and Treatments
To effectively combat obesity, a multi-faceted approach is essential. While BMI and waist circumference are widely used, they offer limited insight into an individual’s true health risks. Newer measurements like body fat percentage and advanced treatments like GLP-1 agonists show promise in treating obesity. Combining lifestyle modifications with tailored pharmacotherapy can help individuals achieve sustainable weight loss and reduce the risk of obesity-related diseases.
References
- Bays, H.E., 2021. Semaglutide: A GLP-1 receptor agonist for weight management in obesity. Diabetes, Obesity & Metabolism, 23(10), pp.2250-2263.
- Bray, G.A., Kim, K.K. and Wilding, J.P., 2017. Obesity: A chronic relapsing progressive disease process. Obesity Reviews, 18(7), pp.715-723.
- Flegal, K.M., Graubard, B.I., Williamson, D.F. and Gail, M.H., 2005. Excess deaths associated with underweight, overweight, and obesity. JAMA, 293(15), pp.1861-1867.
- Garrow, J.S. and Webster, J., 1985. Quetelet’s index (W/H2) as a measure of fatness. International Journal of Obesity, 9(2), pp.147-153.
- World Health Organization (WHO), 2020. Obesity and Overweight. [online] Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 10 Sep. 2024].
**Disclaimer**: The information in this article is intended for informational purposes only and should not be considered medical advice. Always consult a healthcare professional for diagnosis and treatment of medical conditions.


15 Comments